Our purpose is to make
a difference for patients
Immatics is a clinical-stage biopharmaceutical company active in the discovery and development of T cell redirecting immunotherapies for the treatment of cancer. Immatics is sponsoring several clinical trials to deliver the power of T cells to patients with cancer. The purpose of these trials is to develop innovative immunotherapies aimed at targeting a patient’s tumor selectively and effectively.
If you are a physician or patient and would like more information on our clinical trials, please contact: medinfo@immatics.com
You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More Information
You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationClinical
Trials
The following clinical trials are currently open and recruiting patients:
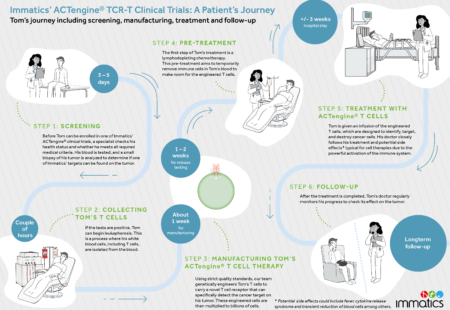
SUPRAME (NCT03686124) is a Phase 3, multicenter, open-label, randomized, actively controlled, parallel-group clinical trial to evaluate the efficacy, safety and tolerability of treatment with ACTengine® IMA203 administered at the recommended Phase 2 dose versus investigator’s choice of treatment in patients with previously treated, unresectable or metastatic cutaneous melanoma.
For more information on this clinical trial, click here
The IMA203-101 Phase 1 trial (NCT03686124) is evaluating the safety and tolerability of ACTengine® IMA203/IMA203CD8 products in patients with recurrent and/or refractory solid tumors that express PRAME (preferentially expressed antigen in melanoma).
For more information on this clinical trial, click here
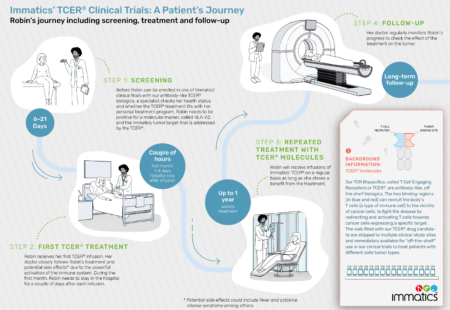
The IMA401-101 Phase 1 trial (NCT05359445) is evaluating the safety, tolerability and initial anti-tumor activity of TCER® IMA401 in patients with recurrent and/or refractory solid tumors. TCER® IMA401 is a TCR Bispecific molecule that targets MAGEA4/8.
For more information on this clinical trial, click here
The IMA402-101 Phase 1 trial (NCT05958121) is evaluating safety, tolerability and anti-tumor activity of TCER® IMA402 in patients with recurrent and/or refractory solid tumors. TCER® IMA402 is a TCR Bispecific molecule that targets PRAME (preferentially expressed antigen in melanoma).
For more information on this clinical trial, click here
Patient
information
If you are a patient and interested participating in one of Immatics’ clinical trials, please speak to your treating physician.
To learn more about clinical trials, reach out to medinfo@immatics.com or click here.