Next-Generation
ACT at a glance
Immatics is committed to developing its ACTengine® programs with the goal of delivering the potential benefits of its innovative science to cancer patients as soon as possible. At the same time, in order to achieve the best outcomes for cancer patients in the longer term, Immatics strives to enhance the tolerability, potency and ease of use of its product candidates. To accomplish these goals, Immatics has taken the following steps:
While our first-generation TCR-T approach demonstrated objective responses across several solid tumors in a Phase 1a clinical data interim read-out, the effective treatment of certain solid tumor indications, as well as maintenance of an anti-tumor attack, may require a second-generation of engineered T cells with enhanced anti-tumor activity. Immatics is actively investigating multiple next-generation strategies to further enhance the potency of TCR-T product candidates.
Aside from improving commercially compatible manufacturing of autologous ACT, Immatics aims to further increase commercial attractiveness and to reach patients more quickly with its next-generation off-the-shelf cell therapy approach, ACTallo®.
For the success of immunotherapies in solid tumors, the complex tumor microenvironment is regarded as one of the biggest challenges. We believe that targeting the tumor stroma could provide a novel approach for the treatment of many solid tumors.
We aim to reduce the likelihood for tumors to evade immunotherapy and prolong durability of clinical responses. In addition to advancing the T cell characteristics, we aim to address this challenge by targeting tumor cells with more than one product candidate. We expect to take the first step towards multi-TCR-T immunotherapy through treatment of patients by applying a combination therapy approach of both anti-tumor and anti-tumor stroma ACTengine® product candidates after clinical proof-of-concept for the individual TCRs.
Immatics believes that its pool of more than 200 prioritized cancer targets combined with its capability to discover and develop the right TCRs generates a long-term and offers a possibly unique foundation to develop cancer treatments for almost any patient at any disease stage in an efficient and cost-effective manner.
Harnessing
the Anti-tumor Potency of CD4 T cells
To further enhance anti-tumor activity and achieve long-lasting clinical responses, several next-generation approaches, like co-transduction of a CD8 co-receptor to engage CD4 T cells, are being pursued in the field of adoptive cell therapies. CD4 T cells are multifaceted in their anti-tumor responses and can not only modulate other immune cell activity through CD4-derived cytokines and chemokines but can also mediate direct cytotoxicity. IMA203CD8 represents our second-generation product candidate targeting PRAME. In contrast to IMA203, IMA203CD8 engages not only CD8 T cells but also CD4 T cells via co-transduction with a CD8 co-receptor (CD8αβ) which plays an important role in T cell antigen recognition and activation. This could further enhance depth and durability of anti-tumor response and potentially improve the clinical outcome of our TCR-T therapies in patients with solid cancer.
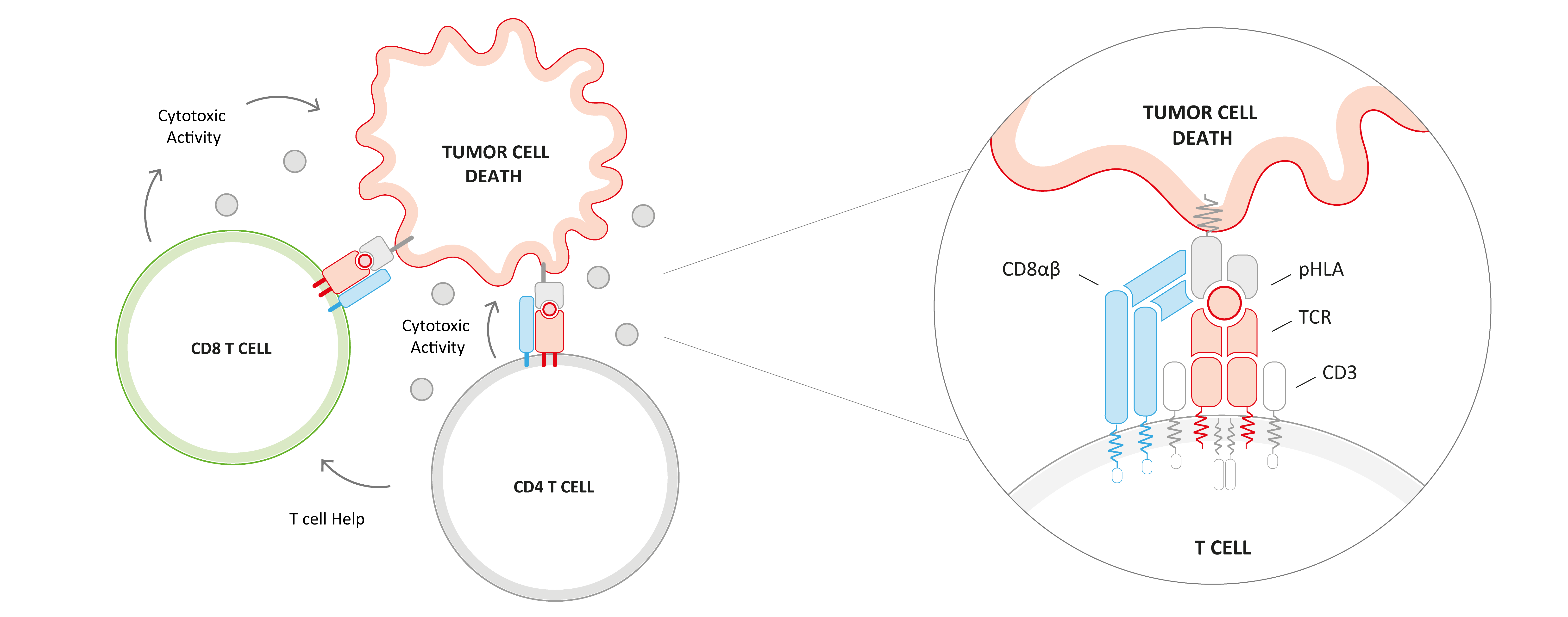
We developed a proprietary lentiviral vector to engineer CD4 and CD8 T cells with the PRAME-specific IMA203 TCR and a CD8αβ construct. CD8αβ showed functional superiority over multiple other CD8 constructs tested in preclinical experiments.
ACTallo®
Off-the-shelf Adoptive Cell Therapy
ACTallo® is Immatics’ proprietary allogeneic, off-the-shelf adoptive cell therapy platform based on gamma delta T cells sourced from healthy donors.
We believe that gamma delta T cells are well suited for an off-the-shelf cell therapy approach, because gamma delta T cells:
are abundant in the peripheral blood
show intrinsic anti-tumor activity
naturally infiltrate solid tumors & correlate with favorable prognosis
are HLA-independent, thus do not cause graft-vs-host disease in allogeneic setting
can be expanded to high numbers in a cGMP-compatible manner
can be effectively redirected using αβ TCR or CAR constructs
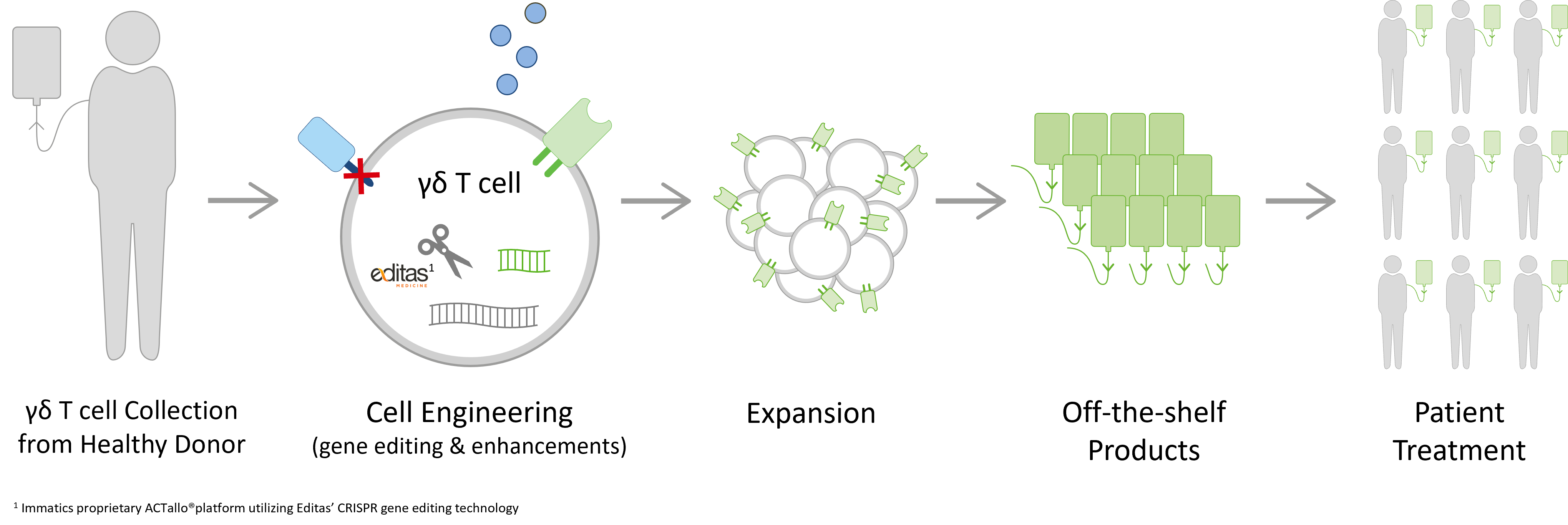
Our manufacturing process is designed to create hundreds of doses from one single donor leukapheresis. The ACTallo® process engineers gamma delta T cells with chimeric antigen receptors (CARs) or T cell receptors (TCRs), thus accessing cancer cell surface targets as well as intracellular proteins that are presented as peptides on the surface of the cancer cell. This enables the redirection of gamma delta T cells to cancer cell targets. ACTallo® products will be available off-the-shelf for patient treatment without the requirement for personalized manufacturing. Since these T cells originate from healthy individuals, they are not reliant on the potentially encumbered immune system of the cancer patient.
In our strategic collaborations with Bristol Myers Squibb and Editas Medicine, we aim to conduct research on and develop next-generation allogeneic gamma delta TCR-T/CAR-T programs with enhanced persistence, safety and potency by combining our proprietary ACTallo® platform with Bristol Myers Squibb’s next-gen technologies and Editas Medicine’s CRISPR gene editing technology.
Targeting
Tumor Stroma
The rigid stroma and the immunosuppressive microenvironment of solid tumors play a crucial role in tumor initiation, progression and metastasis by providing a defensive layer against the body’s immune system and pose a significant challenge for T cell accessibility and activity. Targeting this compartment could provide a novel approach for the treatment of many solid tumors either as single attack or as part of a next-generation multi-TCR-T concept targeting both tumor and stroma simultaneously. Immatics’ ACTengine® program IMA204 is directed against COL6A3 exon 6, a novel tumor stroma target identified and validated by Immatics’ XPRESIDENT® technology platform. COL6A3 exon 6 is highly expressed in the stroma of a variety of solid tumors. Thus, IMA204 is designed to disrupt the tumor’s protective microenvironment in order to make a therapeutic impact.

