Product
Pipeline
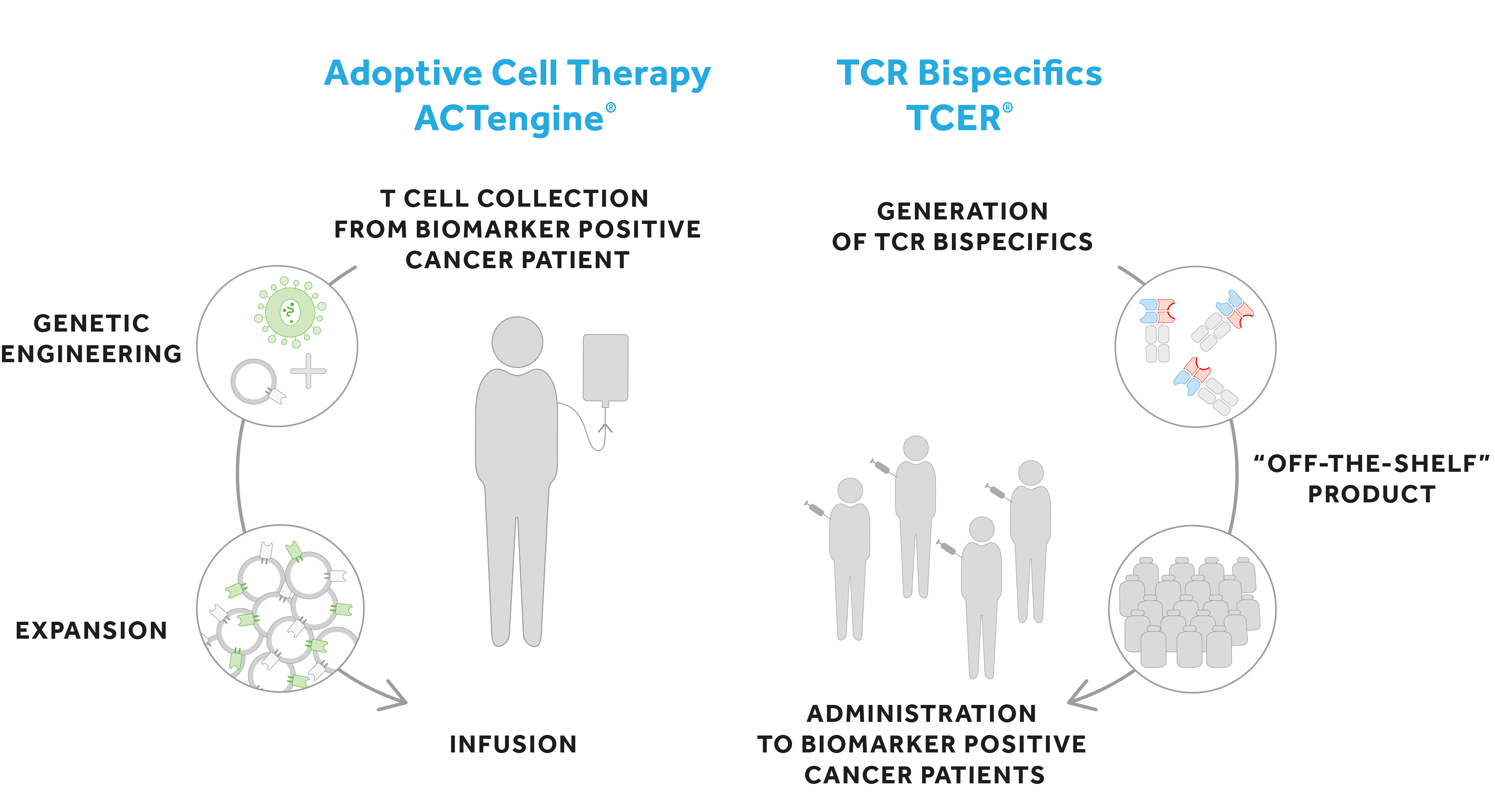
Immatics is developing targeted TCR-based immunotherapies with an emphasis on treating solid tumors through two distinct therapeutic modalities: Adoptive Cell Therapies (ACT) consisting of autologous (ACTengine®) or allogeneic (ACTallo®) product classes and antibody-like TCR Bispecifics, also called T cell Engaging Receptors (TCER®).
Each therapeutic modality is designed with distinct attributes to produce the desired therapeutic effect for patients at different disease stages and with different types of solid tumors:
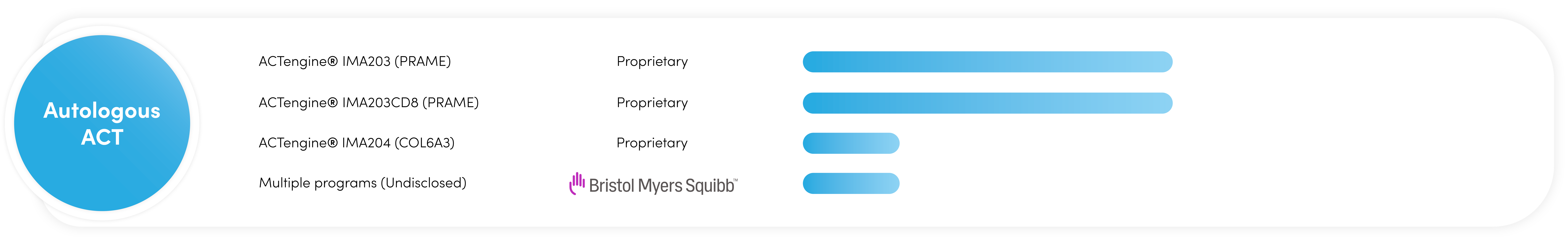
- Adoptive Cell Therapy: Immatics’ clinical product class ACTengine® is a personalized approach in which the patient’s own T cells (autologous) are genetically modified to express a novel proprietary TCR, an approach also known as TCR-T (T cell receptor-engineered T cell). The modified T cells are then reinfused into the patient to specifically engage with the tumor. The product class ACTallo® is advancing the ACT concept beyond individualized manufacturing and is being developed to generate a next-generation of cell therapies, allogeneic “off-the-shelf” cell therapies.
- TCR Bispecifics (TCER®) are engineered “off-the-shelf” biologics designed to bind patients’ circulating T cells and move them into close proximity of the cancer cells to destroy them. A TCER® molecule consists of a portion of the TCR which directly recognizes cancer cells and a T cell recruiter domain which recruits and activates the patient’s T cells.
In addition to our proprietary pipeline, we are also collaborating with world-leading partners to develop additional therapeutic programs covering ACT and Bispecifics. In these partnerships, Immatics seeks to evaluate and develop programs based on novel Immatics-derived targets in a variety of immunotherapeutic approaches.
From its research and development origins in Tübingen, Germany, to its cell therapy center in Houston, Texas, Immatics’ global team is committed to developing and advancing its therapeutic pipeline and collaboration programs to address significant unmet medical needs in oncology.