Engineered Adoptive Cell Therapies
ACTengine®
Our proprietary ACTengine® manufacturing process, timeline, capabilities and facility support late-stage clinical and commercial cell therapy development and supply.
Manufacturing
Process
Autologous ACTengine® TCR T cell therapies (TCR-T) are manufactured specifically for each patient.
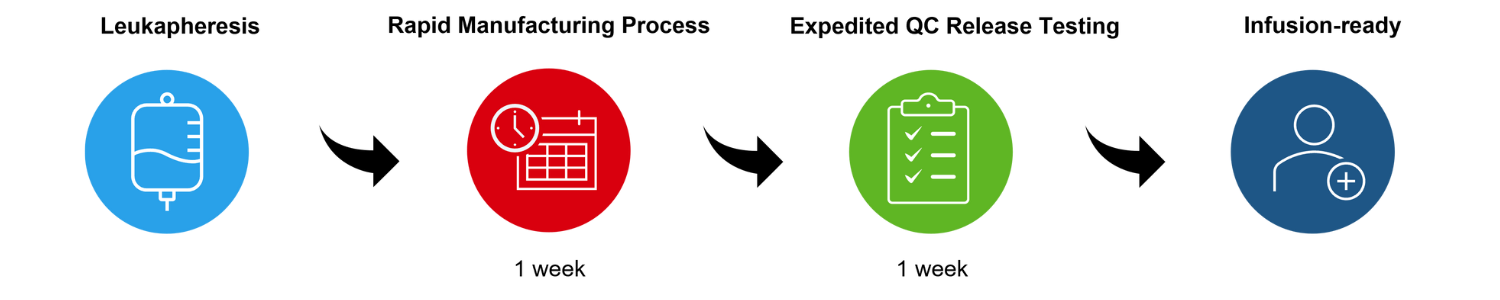
These therapies are derived from a patient’s leukapheresis—a procedure in which the white blood cells, including T cells, of a patient are isolated from their blood. During the cell product manufacturing, T cells are then engineered to carry a novel T cell receptor (TCR) that is designed to specifically detect the cancer target on the patient’s tumor.
The manufacturing process is completed within seven days, followed by seven day quality control (QC) release testing period.
Our manufacturing process generates TCR T-cells that have been shown to achieve a high rate of objective responses, infiltrate the patient’s tumor and function in a challenging solid tumor microenvironment.

Our state-of-the-art ~100,000 sq. ft. R&D and GMP manufacturing facility is situated in the Houston Metropolitan Area, an area with an outstanding talent pool specialized in cell therapy development and manufacturing.
The facility was built with a modular design for efficient and cost-effective scalability. It will serve early-stage and registration-directed clinical trials as well as planned commercial supply.
Through in-house manufacturing and QC testing, we aim to better control the manufacturing process, shorten turnaround time, ensure the manufacturing success rate and quality of the product and achieve potential cost efficiencies. This includes optimizing manufacturing capacity through scalability to create a competitive and profitable commercial cell therapy product.

