TCER®
IMA402
as Monotherapy
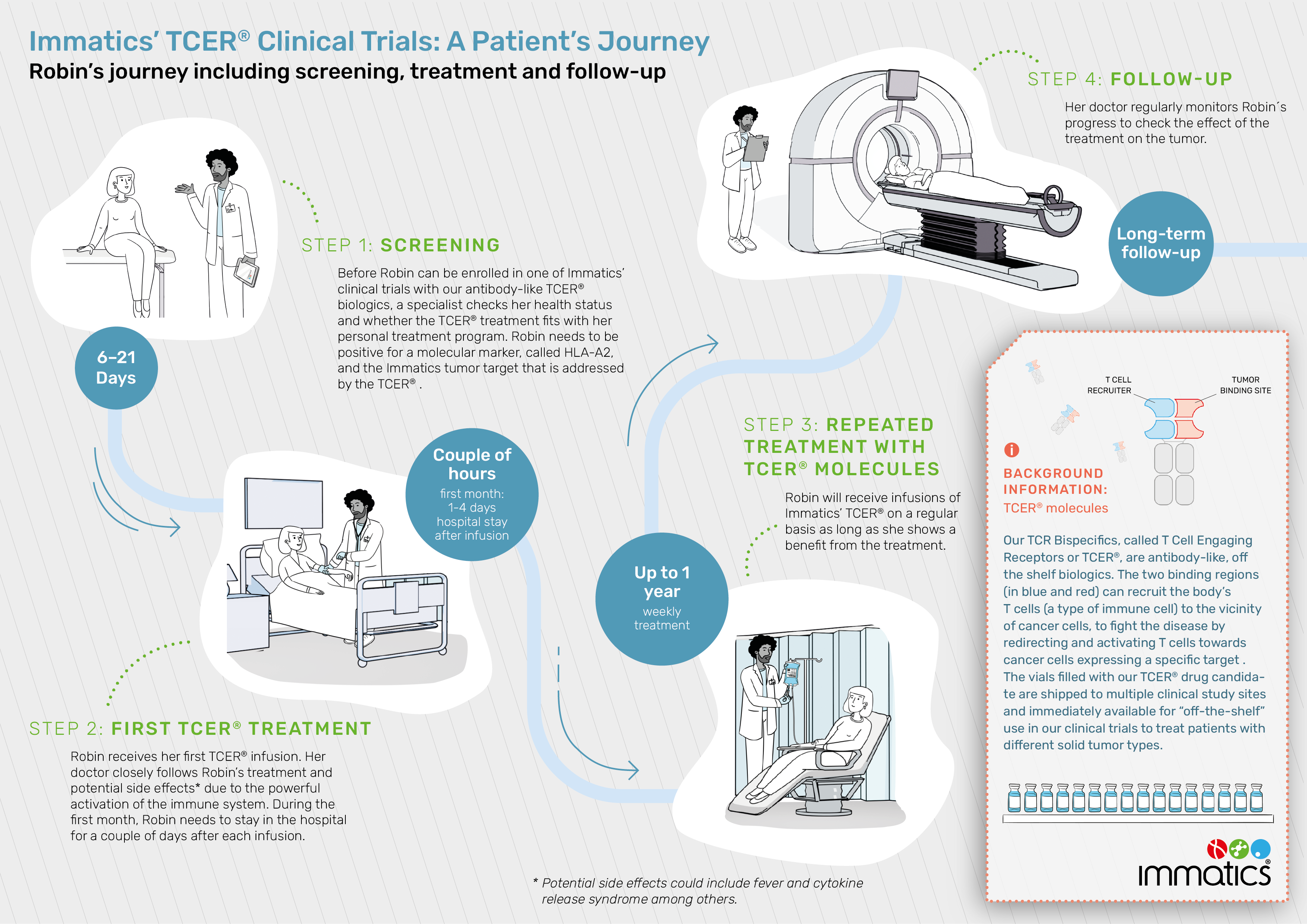
Bispecific T cell engaging receptors (TCER®) are off-the-shelf biologics that leverage the body’s immune system by redirecting and activating T cells towards cancer cells expressing specific tumor targets. The design of these novel biologics allows any T cell in the body to become activated and attack the tumor, regardless of the T cells’ intrinsic specificity.
To learn more about clinical trials, please click here: Patient engagement through education – EUPATI
To learn more about the TCER® technology, please click here: TCR Bispecifics – Immatics
Clinical Trial
Overview
IMA402-101 (NCT05958121) is a Phase 1/2 first-in-human clinical trial to evaluate the safety, tolerability and anti-tumor activity of Immatics TCER® IMA402, a bispecific T cell engaging receptor molecule (TCER®), in patients with recurrent and/or refractory solid tumors. The therapeutic approach is targeting PRAME (preferentially expressed antigen in melanoma)-positive tumors. The target PRAME is expressed in a broad range of solid cancer patients.
IMA402
An antibody-like biologic
IMA402-101 is an investigational therapy that is being tested in clinical trials and has not been approved by the FDA or any other agency for any disease.
Objectives
There are several clinical trial objectives, some of which aim to determine:
- Is the treatment with TCER® IMA402 safe and are potential side effects transient and manageable?
- Has TCER® IMA402 anti-tumor-effects and is able to shrink the patients’ tumor?
Side effects could include fever and cytokine release syndrome among others.
Eligibility
Criteria
A clinical trial is designed to explore efficacy and safety of experimental therapies. If you are a patient and interested participating in one of Immatics’ clinical trials, please speak to your treating physician to discuss the risks and benefits of such a treatment and whether it is fitting in your personal treatment plan.
YOU MAY QUALIFY FOR THE CLINICAL TRIAL IF:
- You are ≥ 18 years of age
- You have a histologically and pathologically confirmed advanced and/or metastatic solid tumor
- You have a blood type of HLA phenotype: HLA-A*02:01 positive
The Clinical Trial
IMA402-101
If you meet these key eligibility criteria, you may be eligible to participate in this clinical trial. There are other additional eligibility criteria that can only be assessed by a clinical trial physician.
Further details for healthcare providers can be accessed below:
IMA402 TCER® in Recurrent and/or Refractory Solid Tumors – ClinicalTrials.gov