Developing
Adoptive
Cell Therapies
Immatics’ clinical ACTengine® programs are based on genetically engineering a patient’s own, autologous T cells with novel TCRs designed to recognize the cancer target on the tumor. The engineered T cells (TCR-T) aim to induce a robust and specific anti-tumor attack to fight the cancer.
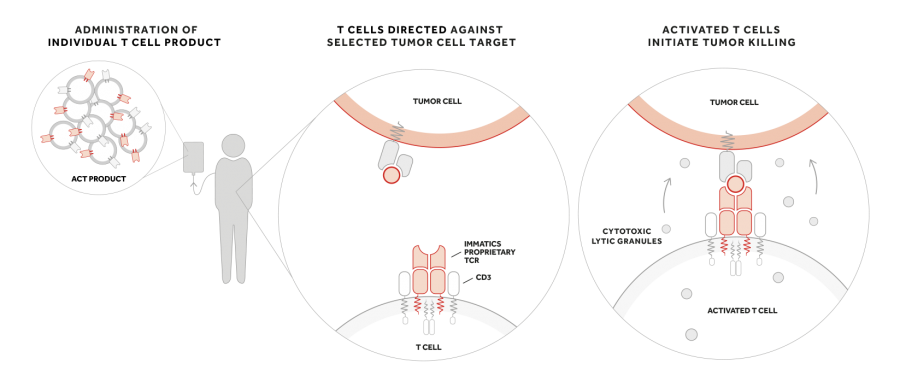
Upon infusion of an ACTengine® product, T cells “equipped” with the cancer target-specific TCR are supposed to bind to the specific target on the tumor. Subsequent activation of the T cell induces release of cytotoxic granules that might ultimately lead to tumor killing.
ACTengine®
at a glance
Immatics’ clinical product class ACTengine® is a personalized approach in which the patient’s own T cells (autologous) are genetically modified to express a novel proprietary TCR, an approach also known as TCR-T (T cell receptor-engineered T cell). The modified T cells are then reinfused into the patient to specifically engage with the tumor. The product class ACTallo® is advancing the ACT concept beyond individualized manufacturing and is being developed to generate a next-generation of cell therapies, our allogeneic “off-the-shelf” cell therapies.
Expanded target space compared to CAR-T
ACTengine® TCR-T product candidates target tumor-associated peptides presented by HLA-molecules on the tumor cell surface. Most relevant solid cancer targets are of intracellular nature and can only be accessible by TCR-based approaches.
TCRs with desirable affinity and specificity
ACTengine® TCRs identified via the XCEPTOR® TCR discovery platform and characterized via XPRESIDENT® guided on- and off-target toxicity screening show desirable affinity and high specificity for their cancer cell target. Immatics’ competitive advantage to other TCR-T approaches is our ability to combine the most suitable target (via XPRESIDENT®) with the right TCR (via XCEPTOR®).
Active at physiological levels of target expression
Immatics believes that ACTengine® TCR-T product candidates are highly potent and capable of inducing the killing of tumor cells that present the target at physiological target copy numbers identified by quantitative mass spectrometry and TCR validation.
Optimized manufacturing
Immatics’ late-stage clinical cell therapy development is supported by its differentiated manufacturing related to timeline, capabilities and facilities. Anzul-cel (anzutresgene-autoleucel, IMA203) cell therapy products are manufactured within 7 days, followed by a 7-day QC release testing at a success rate of >95% to reach the target dose.
Clinical trials
Anzu-cel is currently being evaluated as a monotherapy in a Phase 1 clinical trial in patients with solid tumors expressing PRAME, such as cutaneous melanoma. An anzu-cel registration-enabling randomized controlled Phase 3 trial, “SUPRAME,” is planned to commence in December 2024. Anzu-cel is also currently being evaluated in Phase 1 IMA203CD8 (GEN2) monotherapy, where IMA203 engineered T cells are co-transduced with a CD8αβ co-receptor.
Anzu-cel TARGETING PRAME
Anzu-cel is Immatics’ most advanced TCR-based autologous cell therapy that is directed against an HLA-A*02-presented (human leukocyte antigen) peptide derived from preferentially expressed antigen in melanoma (PRAME), a protein frequently expressed in a large variety of solid cancers. PRAME is homogeneously and specifically expressed in tumor tissue and Immatics’ PRAME peptide is present at a high copy number per tumor cell.
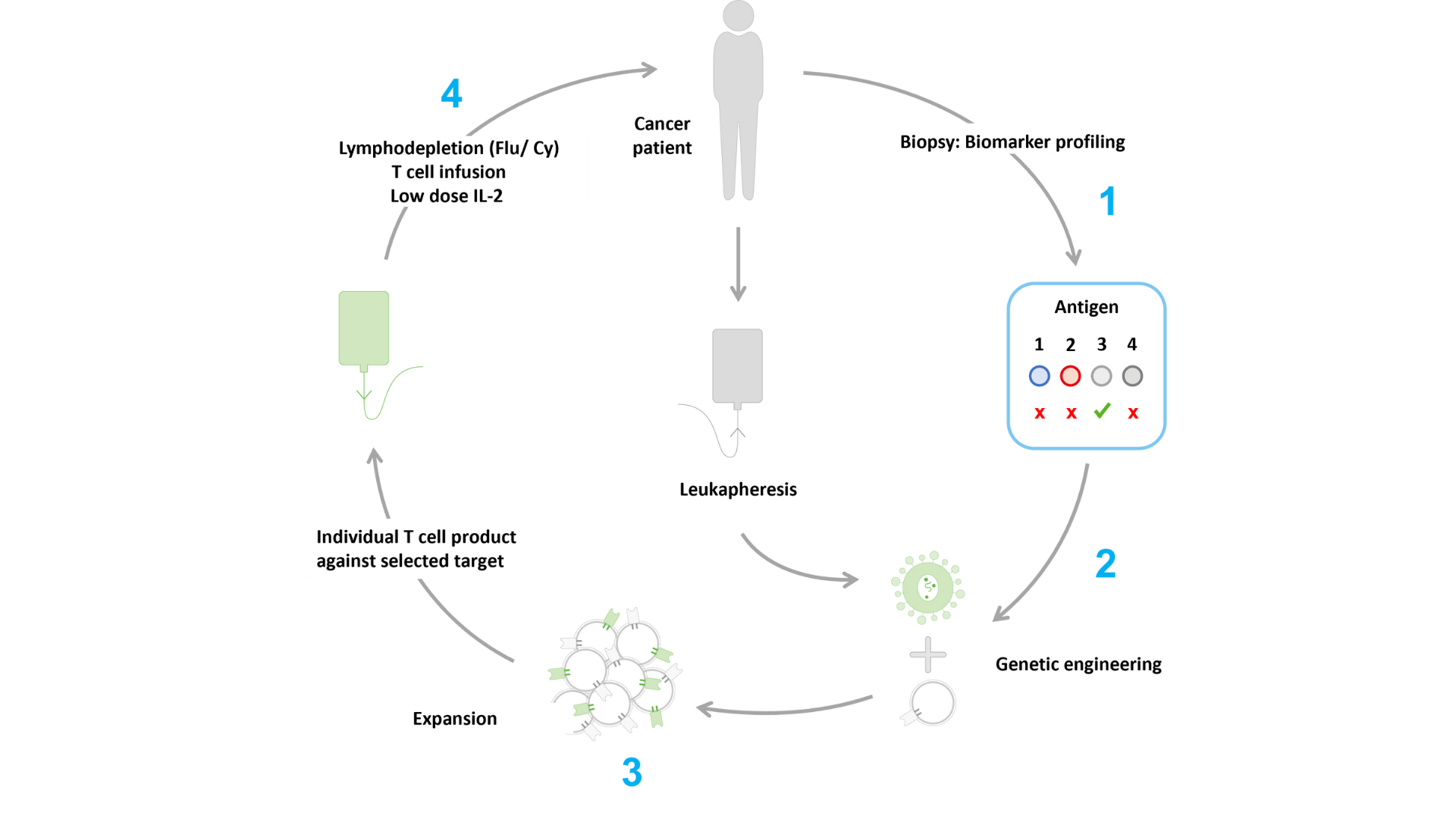
There are several reasons why a patient’s own T cells are often not able to protect the body against cancer. These include the unavailability of activated tumor antigen specific T cells or the insufficient affinity of endogenous target-specific TCRs to properly activate the T cell and destroy the tumor. Immatics’ approach to solving these problems is to engineer autologous T cells with a well characterized and potent TCR, which is the underlying principle of its ACTengine® approach.
ACTengine® is based on genetically engineering a patient’s own T cells with a novel TCR designed to recognize the cancer target that is identified by Immatics’ target discovery platform XPRESIDENT®. (1) The target of interest is first confirmed on a patient’s tumor by Immatics’ proprietary IMADetect® companion diagnostic device candidate. (2) By lentiviral transduction, a patient’s autologous T cells are equipped with a target-specific TCR developed by Immatics. (3) The engineered T cells are then expanded in vitro and the ACTengine® product is ready for infusion into the patient. (4) The patient will receive a pre-treatment, called lymphodepletion, which aims to prepare the patient’s body for the infusion of ACTengine® T cells. By temporarily removing immune cells in the patient’s blood we aim to make room for the engineered T cells and help them to engraft and expand to numbers large enough to fight the tumor. Following infusion, a low dose of IL-2, an immune system signaling molecule, is administered for 14 days to further enhance persistence of the infused engineered T cells.
