ACTengine®
IMA203
as Monotherapy or in Combination with Nivolumab in Recurrent and/or Refractory Solid Tumors
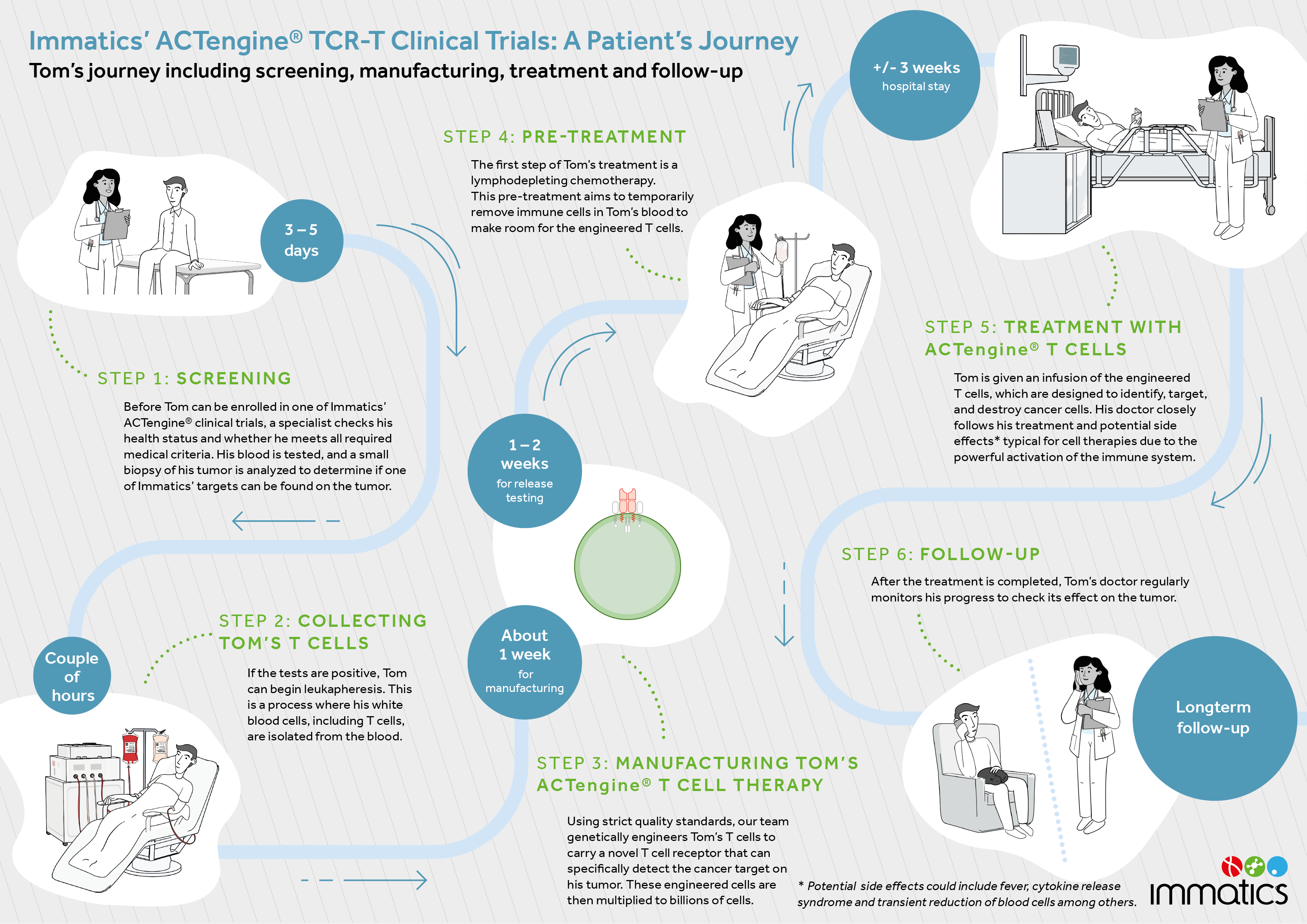
ACTengine® is a personalized therapy in which a patient’s own T cells (immune cells) are collected, genetically modified to recognize and attack cancer tumors, and then reinfused back to the patient as their treatment.
To learn more about clinical trials, please click here: Patient Engagement Through Education – EUPATI
To learn more about the ACTengine® technology, please click here: Adoptive Cell Therapies – Immatics
Clinical Trial
Overview
IMA203-101 (NCT03686124) is a Phase 1 first-in-human dose-escalating clinical trial evaluating the safety, tolerability, and initial signs of clinical and biological efficacy of Immatics’ ACTengine® cell therapy IMA203 in patients with recurrent and/or refractory solid tumors. The therapeutic approach is targeting PRAME-positive tumors, which is expressed in a broad range of solid tumors.
IMA203
A cell therapy
IMA203-101 is an investigational therapy that is being tested in clinical trials and has not been approved by the FDA or any other agency for any disease.
Objectives
There are several clinical trial objectives, some of which aim to determine:
- Is the treatment with ACTengine® IMA203 safe and are potential side effects transient and manageable?
- Has ACTengine® IMA203 anti-tumor-effects and is able to shrink the patients’ tumor?
Side effects could include fever and cytokine release syndrome among others.
You are currently viewing a placeholder content from YouTube. To access the actual content, click the button below. Please note that doing so will share data with third-party providers.
More InformationEligibility
Criteria
A clinical trial is designed to explore efficacy and safety of experimental therapies. If you are a patient and interested participating in one of Immatics’ clinical trials, please speak to your treating physician to discuss the risks and benefits of such a treatment and whether it is fitting in your personal treatment plan.
YOU MAY QUALIFY FOR THE CLINICAL TRIAL IF:
- You are ≥ 18 years of age
- You have a histologically and pathologically confirmed advanced and/or metastatic solid tumor
- You have a blood type of HLA phenotype: HLA-A*02:01 positive
The Clinical Trial
IMA203-101
If you satisfy these key eligibility criteria, you may be eligible to participate in this clinical trial. There are other additional eligibility criteria that can only be assessed by a clinical trial physician.
Further details for healthcare providers can be accessed below:
TCR-engineered T Cells in Solid Tumors – Full Text View – ClinicalTrials.gov